Higher risks of flu and COVID-19 for cancer survivors
Older individuals and people with certain health conditions are known to be at higher risk of severe illness if they contract viruses such as flu and COVID-19. This includes people who had certain cancers diagnosed recently and are receiving treatments like chemotherapy.
In the UK there are more than two million cancer survivors. To investigate whether people who had cancer some time ago are also at higher risk from flu and COVID-19 a study was carried out using CPRD data.
Researchers found that survivors from a wide range of cancers are more likely than people in the general population to be hospitalised or die from flu, even several years after their cancer diagnosis. The raised risks were most likely for blood cancer survivors. Because flu and COVID-19 are both respiratory viruses, this suggested that cancer survivors also have a higher risk of severe COVID-19. The study also showed that cancer survivors were more likely to have other diseases that are associated with increased risk of severe COVID-19, such as heart disease, diabetes, respiratory disease and kidney disease.
The findings support the UK policy recommendation to include all blood cancer survivors as one of the priority groups to receive the COVID-19 vaccination. The study findings could also support any work by others to prioritise vaccinations and treatments for longer-term cancer survivors.
Read the research paper: Carreira H, Strongman H, Peppa M, McDonald H, dos-Santos-Silva I, Stanway S, Smeeth L, Bhaskaran K. Prevalence of COVID-19-related risk factors and risk of severe influenza outcomes in cancer survivors: a matched cohort study using linked UK electronic health records data. EClinicalMedicine, Volume 29, 100656, December 2020.
Safety of the MenB vaccine
The UK was the first country to introduce a national meningococcal group B vaccine (MenB vaccine) programme for infants in 2015. The MenB vaccine protects against infection by meningococcal group B bacteria, which are responsible for more than 90% of meningococcal infections in young children.
To check the safety of the vaccine, the Medicines and Healthcare products Regulatory Agency (MHRA) carried out analysis using CPRD data to investigate possible safety concerns reported to the Yellow Card scheme. Researchers looked at more than three million doses given to about 1.29 million infants up to the age of 18 months.
The study assessed reports of fever, reactions to the vaccine, Kawasaki disease, seizures and sudden death to identify any unexpected side effects. Researchers compared the number of reports with the expected number of events based on the usual rates of the disease and the number of children vaccinated. No new safety concerns were identified.
These findings enabled the MHRA to confirm the safety of the vaccine. The UK Joint Committee on Vaccination and Immunisation (JCVI) continues to recommend the vaccine for all infants alongside their routine immunisations at ages 8 weeks and 16 weeks, followed by a booster when they are one year old.
Read the research paper: Bryan P, Seabroke S, Wong J, Donegan K, Webb E, Goldsmith C, Vipond C, Feavers I. Safety of multicomponent meningococcal group B vaccine (4CMenB) in routine infant immunisation in the UK: a prospective surveillance study. The Lancet Child & Adolescent Health, Volume 2, Issue 6, 2018.
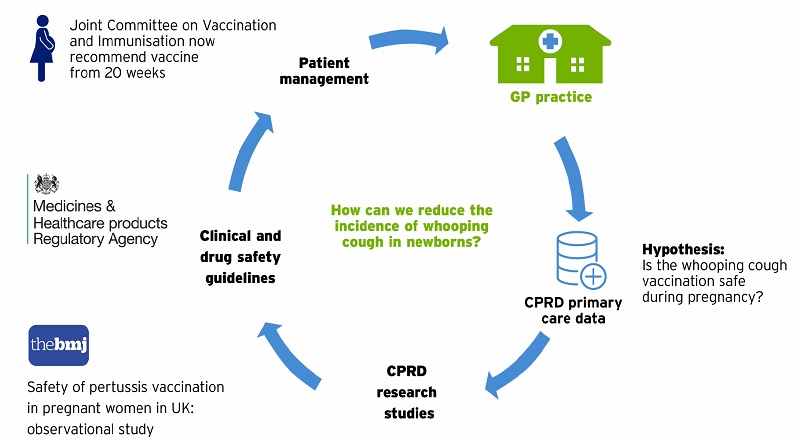
Preventing whooping cough in new-born babies
Whooping cough can cause serious and fatal complications in new-born babies and young children. Babies are routinely vaccinated early, from two months of age, but can still be at risk if a mother catches whooping cough whilst pregnant.
After an outbreak of whooping cough in 2012, a national programme was introduced to give pregnant mothers a vaccine to protect their baby. CPRD data was vital for checking the safety and effectiveness of the vaccine after the programme was introduced. As a result of the research, all GPs and maternity services now routinely vaccinate all pregnant mothers from 20 weeks.
The CPRD database is crucial for investigating the safety and effectiveness of medical treatments during pregnancy. The greater the number of women in our database, either pregnant or not pregnant and of all ages, the more treatments we can investigate.
Read the research paper: Donegan K, King B, Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ. 2014;349:g4219.
Helping doctors to diagnose cancer as early as possible
The National Institute of Clinical Excellence (NICE) regularly reviews new medical research and write guidelines on the best and most up-to-date ways to diagnose and treat patients.
One important decision that doctors must regularly make is when a patient’s symptoms could suggest the possibility of cancer. In this case, NICE guidelines are vital to help doctors decide when to refer patients for further specialist tests. For example, a patient visiting their doctor with blood in their urine may have an early sign of bladder cancer, or, more likely, they may have a bladder infection. NICE guidelines support doctors with the decision of how best to move forward with treatment.
Research using the CPRD database is essential for writing NICE guidelines. It helps researchers to calculate how many patients with a particular symptom go on to be diagnosed with cancer. This allows researchers to attach a ‘level of risk’ to all the symptoms commonly seen in patients visiting their doctor, and helps doctors to know when further investigation is needed.
Confirming the benefit of the flu vaccine for patients with type 2 diabetes
For individuals with type 2 diabetes, catching the seasonal winter flu may increase the risk of heart disease complications such as heart attack, stroke and pneumonia. As a result, doctors currently recommend that individuals with diabetes receive the flu vaccine each year. CPRD data has been used to investigate the benefits of the flu vaccine for preventing admissions to hospital and death due to flu.
Researchers found that individuals who had received the flu vaccine had fewer incidences of stroke and heart failure over a seven-year period. In contrast, individuals with diabetes who had not been vaccinated had a higher chance of death than those who had been vaccinated.
This study was crucial to confirm the benefits of flu vaccination and that the current recommendation for yearly immunisation is worthwhile and accurate. The study also guides doctors as to which patients should receive the flu vaccination as a priority for individual health, and to reduce the number of hospital admissions nationwide.
Read the research paper: Vamos EP, Pape UJ, Curcin V, Harris MJ, Valabhji J, Majeed A, Millett C. Effectiveness of the influenza vaccine in preventing admission to hospital and death in people with type 2 diabetes. CMAJ. 2016 Oct 4;188(14):E342-E351.