The importance of patient records for improving health care
Research is important to the NHS and benefits us all, whether we are fit and healthy or have an illness or condition. Research helps us understand the causes, prevention and treatment of diseases and medical conditions and improves the health care we receive.
Whenever you visit your doctor or use an NHS service, your electronic health record is updated. This record contains important information about your health and wellbeing and describes the care that you have received, which is vital for medical research.
For more than 30 years, GP practices across the UK have chosen to work with CPRD so that anonymised health information from patient records can be used for important medical research such as:
- What causes illness and how to prevent and treat it
- Monitoring the safety of vaccines and medicines
- Understanding possible side effects of treatments
- Assisting the delivery of clinical trials
How patient data is used for medical research
Research using CPRD anonymised patient data has provided evidence for drug safety guidance and improved healthcare delivery. More than 3,000 medical and public health research studies have been published using CPRD data.
See examples of how CPRD anonymised data has been used to benefit patient care and public health.
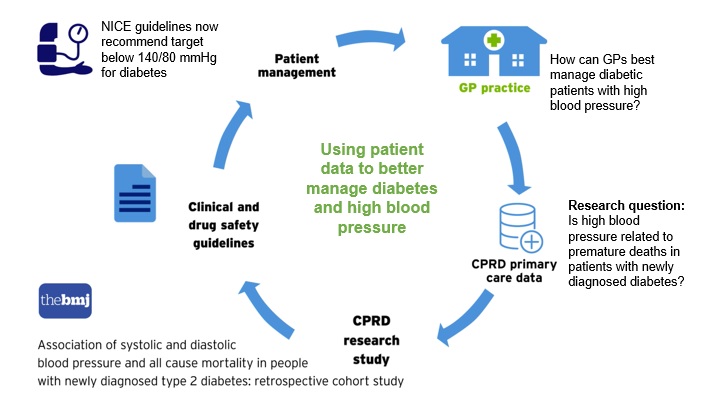
How patient data is used to better manage diabetes and high blood pressure
How organisations use patient data
Researchers wanting to use CPRD data must go through a rigorous review process. Information on the approved studies page shows how CPRD data is being used in public health and medical research studies and which organisations are carrying out these studies including medicines regulators such as the Medicines and Healthcare products Regulatory Agency (MHRA), academic researchers, the NHS and pharmaceutical companies.
Examples of how data has been used include:
- Work by the MHRA to monitor the safety of authorised vaccines, such as COVID-19 vaccines and the MenB vaccine. The MenB vaccine is recommended for babies aged 8 weeks, 16 weeks and 1 year and protects against infection by meningococcal group B bacteria. Find out more here
- A Public Health England study using CPRD data and the CPRD pregnancy register, to assess the effectiveness of vaccinating mothers to protect their babies against flu. The research paper is available here
- Investigating whether having abnormally small red blood cells could indicate the presence of cancer, in academic research funded by Cancer Research UK and NIHR. The research paper is available here
- Academic research examining whether damage to kidneys and eyes may start before people are diagnosed with diabetes. Read a summary of the study on the NIHR website
- In a drug safety study by a pharmaceutical company, to monitor the long-term safety of different drugs used to treat Parkinson’s disease. The research paper is available here
- Studying the impact of COVID-19 on the use of mental health services and on mental illness. The research paper is available here
Protecting your confidentiality and privacy
Protecting patient privacy is essential. This is why CPRD never receives information that identifies patients and only provides anonymised health data to approved researchers. Your confidential information is protected in the following ways:
- You cannot be identified from the information sent to CPRD from your GP practice or from any data CPRD receives from other sources.
- CPRD never receives identifying details, such as your name, address, NHS number, date of birth or medical notes
- Data can only be used for research to improve patient and public health
- CPRD must obtain annual ethics and governance approvals to collect and supply data for research
- Researchers must comply with strict terms and conditions to use anonymised CPRD data for research
The ability to link primary care data to other health datasets enables researchers to have a more complete picture of a patient’s medical history. In England, this data linkage is carried out via NHS England, the statutory body legally permitted to receive identifiable patient data. The linkage process involves NHS England receiving and processing identifiable non-clinical information about a patient, on behalf of CPRD. NHS England only sends anonymised linked data to CPRD.
As an additional step to protecting patient confidentiality, we do not share details of the GP practices that contribute data to CPRD. Find out more about how we protect your confidential information on our Safeguarding Patient Data page and Data protection and processing notice web page.
You can opt out
Because you cannot be identified from the data GP practices send to CPRD, GPs do not need to seek individual patient consent when they share data with CPRD.
However, you can opt out of your patient information being shared for research. If you do not want your GP practice to share your health record, let your doctor know if you live in Scotland, Wales or Northern Ireland. If you are in England you can find out more about the National Data Opt-out.
Opting out of sharing your health records will not affect the care that you receive. However, the Government depends on the data collected by CPRD to monitor drug safety and safeguard public health. If large numbers of patients choose not to share their anonymised health information for research, the health information within the CPRD database will not truly represent the UK population.
CPRD is an official supporter of Understanding Patient Data, an initiative that aims to make uses of patient data more visible, understandable and trustworthy, for patients, the public and health professionals.
There is also information on the Understanding Patient Data website: The national data opt-out.